
Experts Discuss:
Patient Phenotypes Within the Risankizumab PsA Clinical Program
- 14-minute watch
Dr. Ogdie and Dr. Khattri discuss a new analysis of patient phenotypes in PsA from risankizumab clinical trials, and the implications for patient care across different patterns of disease manifestation
Video
The experts

Alexis Ogdie, MD
Rheumatologist
University of Pennsylvania
Philadelphia, Pennsylvania

Saakshi Khattri, MD
Rheumatologist and
Dermatologist
Icahn School of Medicine at
Mount Sinai
New York,
New York
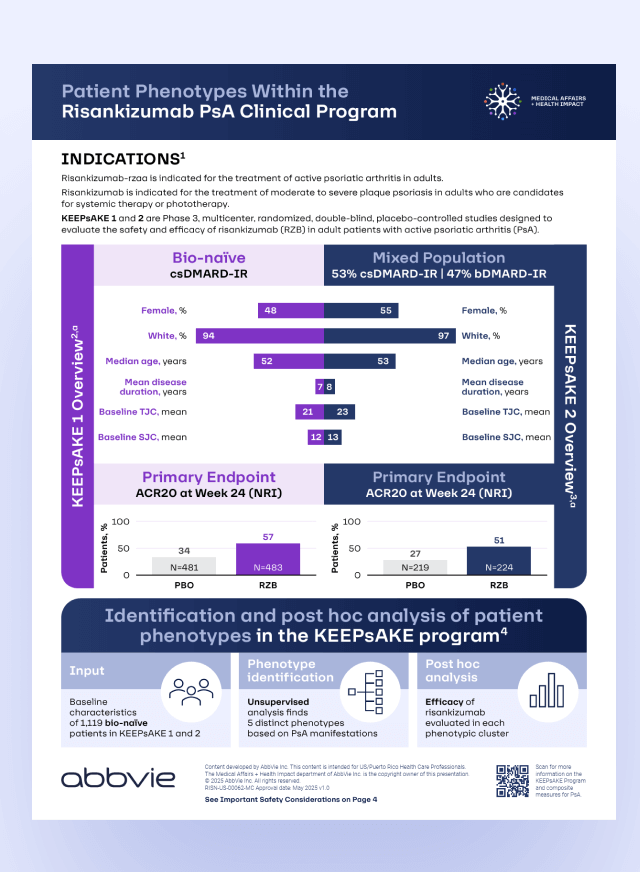
Key information
Post hoc analysis of bio-naïve patients from the KEEPsAKE trials of risankizumab in PsA identified patient clusters that were defined by different patterns of disease manifestation1
Safety in the KEEPsAKE trials
Most common AEs through controlled periods in risankizumab trials
aIncludes: respiratory tract infection (viral, bacterial, or unspecified), sinusitis (including acute), rhinitis, nasopharyngitis, pharyngitis (including viral), tonsillitis; bIncludes: headache, tension headache, sinus headache, cervicogenic headache; cIncludes: fatigue, asthenia; dIncludes: injection site bruising, erythema, extravasation, hematoma, hemorrhage, infection, inflammation, irritation, pain, pruritus, reaction, swelling, warmth; eIncludes: tinea pedis, tinea cruris, body tinea, tinea versicolor, tinea manuum, tinea infection, onychomycosis
Adverse drug reactions occurring in ≥2% of patients in KEEPsAKE 1 (bio-naïve, csDMARD-IR) through Week 24
The overall safety profile observed in participants with PsA treated with risankizumab is generally consistent with the safety profile in participants with plaque PsO, with the addition of hepatic events and hypersensitivity reactions.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; b, biologic; COVID-19, coronavirus disease of 2019; cs, conventional synthetic; DMARD, disease-modifying antirheumatic drug; E, event; HZ, herpes zoster; IR, inadequate responder; MACE, major adverse cardiovascular event; NMSC, nonmelanoma skin cancer; PBO, placebo; PsA, psoriatic arthritis; PsO, psoriasis; PY, patient-year; RZB, risankizumab; TB, tuberculosis; TEAE, treatment-emergent adverse event.
Indications
Important safety considerations
Risankizumab is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, may occur. If a serious hypersensitivity reaction occurs, discontinue risankizumab and initiate appropriate therapy immediately. Risankizumab may increase the risk of infections. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If such an infection develops, discontinue risankizumab until the infection resolves. Evaluate patients for tuberculosis infection prior to initiating treatment with risankizumab. Avoid use of live vaccines in patients treated with risankizumab. The most common adverse reactions (≥1%) are upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
Review accompanying risankizumab-rzaa full Prescribing Information for additional information, visit www.rxabbvie.com or contact AbbVie Medical Information at 1-800-633-9110.
How well does the moderate PsA phenotype discussed in this video align with your clinical experience?
From the data discussed in this video, which is most helpful in informing your treatment selection in PsA?
How frequently do you consider an IL-23 inhibitor for bio-naïve patients with moderate, active PsA?
Resource
Find out more about patient phenotypes in KEEPsAKE trials

- Gossec L et al. ACR Convergence 2024. Abstract 2355.
- Kristensen LE et al. Ann Rheum Dis. 2022;81(2):225-231.
- Kristensen LE et al. ACR Convergence 2022. Abstract 2145.
- Data on file, AbbVie Inc. ABVRRTI73417.
- Kristensen LE, et al. Poster presented at: Fall Clinical Dermatology Conference; October 21-24, 2021; Las Vegas, NV.
- Data on file, AbbVie Inc. ABVRRTI74973.
- Östör A, et al. Poster presented at: Fall Clinical Dermatology Conference; October 21-24, 2021; Las Vegas, NV.
- Östör A et al. Ann Rheum Dis. 2022;81(3):351-358.
- SKYRIZI (risankizumab-rzaa) [package insert]. North Chicago, IL: AbbVie Inc.
- Strober B et al. AAD 2019. Abstract P9876
- Data on file, AbbVie Inc. ABVRRTI68139.